Association between sleep quality in late pregnancy and birth weight
Subathra S¹, Dharani B², Umayal CC³
¹Assistant Professor, ²Post Graduate, ³Associate Professor, Dept of Physiology, Stanley Medical College,
Chennai – 600 001, Tamil Nadu, India
Abstract
Background: Low Birth Weight is a major public health problem since birth weight determines the health and development of a child. There are few studies that have reported that poor sleep quality in pregnancy is associated with adverse fetal outcome. Low Birth Weight is defined by World Health Organization as a neonate weighing less than 2500gm at birth irrespective of the gestational age. Low Birth Weight is the most common adverse fetal outcome that may lead to Perinatal mortality and morbidity. As per the National sleep foundation, about 78% women report sleep disturbance in pregnancy especially during third trimester.
Aim: To find out the association between the Sleep quality in late pregnancy and birth weight.
Materials and methods: Using Pittsburg Sleep Quality Index (PSQI) questionnaire, Sleep quality for the last one month of pregnancy was assessed and Birth weight of the newborn was recorded from mother’s hospital documents in 90 Postnatal mothers who had uneventful Antenatal Period. Association between the sleep quality in late pregnancy and the Birth weight was assessed.
Results: The data obtained were analyzed using Statistical Package for Social Sciences (SPSS) version 20. 90 antenatal mothers whose age ranged from 18 to 40 years Participated in the study (M=24.94years, SD=4.42). Correlation coefficient between PSQI score of sleep quality and the birth weight is -0.05.
Conclusion: The sleep quality in late pregnancy is inversely related to the birth weight which indicates that poor sleep quality during late pregnancy is a risk factor for Low birth weight. Hence, antenatal interventions to improve the sleep quality during pregnancy can be suggested for better fetal outcome.
Keywords: low birth weight, pregnancy, sleep quality
Corresponding Author ::
Dr. B. Dharani, Postgraduate,
Department of Physiology,
Stanley Medical College,
Chennai – 600001
Telephone: +91 8610263796
E-mail: [email protected]
Introduction: Birth weight determines the health and development of a child. Hence, Low birth weight can lead to developmental disorders. Low birth weight is defined by WHO as a neonate weighing less than 2500gm at birth irrespective of the gestational age. Low birth weight is the most common adverse fetal outcome that may lead to perinatal mortality and morbidity. Low birth weight is associated with respiratory, digestive and neurological complications. Low birth weight is because of problems with the placenta, the mother’s health or the baby’s condition. As per the American Pregnancy Association, a fetus gains weight of around 2 pounds by 27weeks, 4 to 41/2 pounds by 32weeks and 63/4 to 10pounds by term. Hence during the late pregnancy, the fetus gains the maximum weight.
As per the National sleep foundation, about 78% of ante natal mothers report sleep disturbance in pregnancy especially during third trimester1. Many physiological, hormonal and physical changes occur during pregnancy. These are gestational weight gain, pregnancy-associated nasopharyngeal edema, low functional-reserve capacity and frequent arousals from sleep18,19. These changes that are occurring during the third trimester of pregnancy can alter the sleep patterns20,21. During the third trimester, most common sleep problems are nocturnal waking to void, heartburns, restless sleep due to fetal movements, restless leg syndrome and obstructive sleep apnea. These factors lead to disrupted sleep.
Hence during pregnancy, pregnant women experience typical characteristics of sleep loss such as short sleep duration, poor sleep quality, poor sleep efficiency with an increase in time spent awake at night and insomnia. It was found that pregnant women with sleep problems also experience longer labor duration and they are more likely to undergo caesarean section. Pregnancy is a period of increased stress. Maternal stress was found to be associated with increased perinatal mortality and morbidity.
Many studies have explained sleep-disordered breathing or snoring in pregnancy and its association with gestational hypertension23,24, gestational diabetes25 and preterm birth26,27. Severe snoring and sleep deprivation in late pregnancy are commonly associated with adverse birth outcomes which are low birth weight, fetal growth retardation, and preterm birth.
There are few studies that have reported that poor sleep quality in pregnancy is associated with adverse fetal outcome. Sleep disorders during pregnancy cause increased oxidative and inflammatory stress response2-4. These are the key factors leading to preterm birth and low birth weight2-4. Hence this study was done to find out the association of sleep quality in late pregnancy and the birth weight.
Aim and objectives:
1. To evaluate the Sleep quality in late Pregnancy by using Pittsburg Sleep Quality Index (PSQI) questionnaire in the Postnatal period.
2. To record the birth weight of the newborn.
3. To find out the association between the sleep quality in late pregnancy and the birth weight.
Materials and Methods: This was a Cross-sectional study done among 90 postnatal mothers of department of Obstetrics and Gynecology and the duration of study was 3 months.
Inclusion criteria:
Postnatal mothers who had uneventful Antenatal period.
Exclusion criteria:
Postnatal mothers who had;
• Postpartum complications,
• History of maternal diseases during pregnancy,
• Caesarean section due to maternal diseases,
• Placental pathology,
• Multiple pregnancy,
• TORCH infection,
• Fetal malformations & chromosomal abnormality,
• History of smoking, Alcohol intake.
Data collection was done after obtaining Institutional Ethical Committee clearance. After giving complete information about the study, informed and written consent was obtained from the participants. The participants were assured of the confidentiality. Using a validated semi structured proforma, a detailed Antenatal history was obtained. A detailed clinical examination was done. Then the participants were administered the Pittsburg Sleep Quality Index (PSQI) questionnaire.
Pittsburg Sleep Quality Index (PSQI) questionnaire is an effective tool to measure the quality and patterns of sleep during pregnancy. It is used to differentiate ‘poor’ from ‘good’ sleep quality. It has seven components that assesses subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications and daytime dysfunction. It has a score range of 0-21, out of which a score of more than 5 is suggestive of ‘poor’ sleep quality.
Using Pittsburg Sleep Quality Index (PSQI) questionnaire, Sleep quality for the last one month of the pregnancy was assessed and Birth weight of the newborn was recorded from mother’s hospital documents. Association between the sleep quality in late pregnancy and the Birth weight was assessed.
Statistical analysis: The data obtained were analyzed by using Statistical Package for Social Sciences (SPSS) version 20. The categorical variables were expressed in percentage. Correlation between Sleep quality and birth weight was done by Pearson’s rank correlation(r).
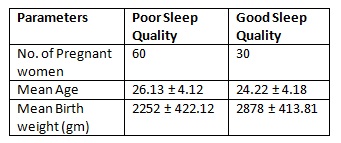
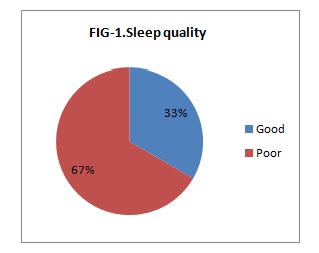
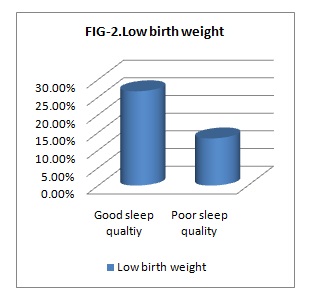
Results: The data obtained were analyzed using SPSS software version 20. The average age of Participants was 24.94 years with an SD of 4.42. Of 90 participants, 66.66% (n=60) had poor sleep quality & 33.33% (n=30) had good sleep quality. Among those with Poor sleep quality 26.66% had Low birth weight & among those with Good sleep quality only 13.33% had Low birth weight babies.
Table 1 represents the baseline characteristics of the participants. Using Pearson’s correlation between PSQI score of sleep quality & the birth weight, the r value is -0.05. Thus, the sleep quality in late pregnancy is negatively correlated with the birth weight.
Discussion: Birth weight predicts the child’s future health and development. Low birth weight contributes to about 60% of Infant mortality globally. Low birth weight increases the risk of long-term complications like Cerebral palsy and developmental delay. They can develop hypoglycemia, hypothermia, hypercoagulability, hyperbilirubinemia, hypotension, necrotizing enterocolitis and Respiratory distress syndrome during the neonatal period.
Sleep disturbances are more prevalent among pregnant women, especially during the third trimester. Increased frequency of micturition, back pain and leg pain are the common complaints reported by pregnant women as cause of sleep disturbances. These pregnancies related physical symptoms or discomfort are common experiences of pregnancy.
High estrogen level during pregnancy tend to cause upper airway narrowing22. This could predispose to snoring and obstructive sleep apnea. Sleep disordered breathing (eg. Snoring, Nocturnal apnea and daytime sleepiness) was found to be associated with fetal growth restriction6. The frequency of sleep disordered breathing was markedly increased during the third trimester7. The prevalence of snoring and sleep deprivation in late pregnancy was found to be between 10% to 30% 21,23,29. Katerina Micheli et al found in their study that women with severe snoring during late pregnancy are at high risk for low birth weight and fetal growth restricted neonates28. Sleep deprivation was found to be associated with increased risk of preterm births.
Our study found an increased incidence of insomnia symptoms among pregnant women in late pregnancy. Ivan D.Sedov in their study found that sleep quality may be stable through first and second trimesters with lower sleep quality evident in third trimester9. Jodi A.Mindell et al study found that sleep was highly disrupted during pregnancy because of frequent night waking with an average of two to three times per night for over an hour8. Duration of sleep decreases in the third trimester on average of 6 to 7hours12,13. Insomnia worsens in late pregnancy because of oxytocin which is a wake promoting hormone.
In the present study it is found that poor sleep quality in late pregnancy is associated with low birth weight. This indicates that poor sleep quality in late pregnancy is a risk factor for adverse fetal outcome. Similar findings were observed in G.Bourjeily et al study.
During pregnancy, increased minute ventilation with resulting respiratory alkalosis and increased sensitivity of the respiratory center to carbon dioxide can lead to central sleep apnea33,34. Artal et al in their study observed that during third trimester increased fetal demand and metabolic alterations in the mother combine to produce a 20% to 33% increase in oxygen consumption. Hence, the combination of low functional residual capacity and high oxygen consumption results in a low oxygen reserve. Even a small decrease in maternal oxygen level can lead to decrease in oxygen delivery to the fetus18.
Sleep deprivation leads to low-grade systemic inflammation and increased levels of proinflammatory serum cytokines18,30-32. This results in stimulation of the inflammatory pathway contributed from the mother to the fetus which can lead to preterm births in women with sleep deprivation in late pregnancy30-32. Sleep disturbances in late pregnancy was found to be associated with raised level of Interleukin – 6, C-Reactive Protein and an inflammatory state.
Guopeng Li et al found in their study that greater resilience was associated with better sleep quality10. High resilience maintains the HPA axis at an optimal level of activation which is not high enough to stimulate excess fear and anxiety. By this study only the association between the subjective sleep quality in late pregnancy and birth weight were analyzed. Hence to prove this, future research should include more in-depth evaluation of these physiological factors.
Conclusion: The sleep quality in late pregnancy is inversely related to the birth weight which implies that poor sleep quality in late pregnancy is a risk factor for Low birth weight. Hence, antenatal interventions to improve the sleep quality in pregnancy can be suggested for the better fetal outcome.
Limitations: This study assessed only subjective sleep symptoms and lacks polysomnography data. With the current findings, further studies should include objective measures of sleep quality.
Acknowledgements: Nil.
Conflict of Interest: Nil.
References:
1. Ying Yang, Jing Mao, Zhiying Y, Jie Li, Huimin Zhao and Yueting Liu. Determinants of sleep quality among pregnant women in China: A cross-sectional survey. NursPalliat Care. 2017;2(3):1-5.
2. Fabiana Bernardi, FrancieliGuolo,ThaizeBortolin,FabriciaPetronilho and Felipe Dal-Pizzol. Oxidative stress and inflammatory markers in normal pregnancy and preeclampsia. J ObstetGynaecol Res. 2008;34(6):948-951.
3. Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF III, Petraglia F. Inflammation and pregnancy. Reprod Sci 2009;16(2):206-215.
4. Carl A.Hubel. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med 1999;222(3):222-235.
5. Ekaterina Kamysheva, Helen Skouteris, Eleanor H.Wertheim, Susan J.Paxton, Jeannette Milgrom. A prospective investigation of the relationships among sleep quality, physical symptoms, and depressive symptoms during pregnancy. Journal of Affective Disorders. 2010;123:317-320.
6. G.Bourjeily, C.A.Raker, M.Chalhoub and M.A.Miller. Pregnancy and fetal outcomes of Sleep-disordered breathing. European Respiratory Journal. 2010; 36: 849-855.
7. Francesca L.Facco, MD, Jamie Kramer, MD, Kim H.Ho, BS, Phyllis C.Zee, MD, and William A.Grobman, MD, MBA.Sleep Disturbances in Pregnancy. The American college of Obstetrics &Gynaecologists Journal. 2010;115(1):77-83.
8. Jodi A.Mindell, Rae Ann Cook, JanetaNikolovski. Sleep patterns and sleep disturbances across pregnancy. Sleep Medicine.2015;16:483-488.
9. Ivan D.Sedov, Emily E.Cameron, Shery Madigan, Lianne M.Tomfohr-Madsen. Sleep quality during pregnancy: A meta-analysis. Sleep Medicine Reviews. 2018;38:168-176.
10. Guopeng Li, Linghua Kong, Haiyan Zhou, Xiaofeikang, Yeuyan Fang, Ping Li. Relationship between prenatal maternal stress and sleep quality in Chinese pregnant women: the mediation effect of resilience. Sleep Medicine. 2016;25:8-12.
11. Cristina A Reichner. Insmonia and Sleep Deficiency in Pregnancy. Obstetric Medicine. 2015;8(4):168-171.
12. Hedman C, Pohjasvaara T, Tolonen U, Suhonen-Malm A.S, Myllyla V.V. Effects of pregnancy on mother’s sleep. Sleep Med. 2002;3(1): 37–42.
13. Lee KA, Zaffke ME, McEnany G. Parity and sleep patterns during and after pregnancy. Obstet Gynecol. 2000; 95: 14–18.
14. Michele L.Okun, Martica Hall, Mary E. Coussons-Read. Sleep disturbances increase interleukin-6 production during pregnancy: implications for pregnancy complications. Reprod Sci. 2007;14:560–567.
15. Michele L.Okun, Mary E. Coussons-Read. Sleep disruption during pregnancy: how does it influence serum cytokines?. J Reprod Immunol. 2007;73:158–165.
16. Gabriel Natan Pires, Monica Levy Andersen, MarciaGiovenardi, Sergio Tufik. Sleep impairment during pregnancy: Possible implications of mother-infant relationship. Med Hypotheses. 2010;75(6): 578–582.
17. Wendy A. Hall, Yvonne L. Hauck, Elaine M. Carty, Eileen K. Hutton, Jennifer Fenwick and Kathrinstoll. Childbirth Fear, Anxiety, Fatigue, and Sleep Deprivation in Pregnant Women. JOGNN. 2009;38(5): 567-576.
18. Izci-Balserak B, Pien GW. Sleep-disordered breathing and pregnancy: potential mechanisms and evidence for maternal and fetal morbidity. CurrOpinPulm Med. 2010;16:574–582.
19. Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004; 27:1405–1417.
20. Facco FL, Kramer J, Ho KH, Zee PC, Grobman WA. Sleep disturbances in pregnancy. Obstet Gynecol. 2010;115:77–83.
21. Pien GW, Fife D, Pack AI, Nkwuo JE, Schwab RJ. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep. 2005;28: 1299–1305.
22. Maasilta P, Bachour A, Teramo K, Polo O, Laitinen LA. Sleep-related disordered breathing during pregnancy in obese women. Chest. 2001; 120:1448–1454.
23. Franklin KA, Holmgren PA, Jonsson F, et al. Snoring, pregnancyinduced hypertension, and growth retardation of the fetus. Chest. 2000; 117:137–141.
24. Ursavas A, Karadag M, Nalci N, Ercan I, Gozu RO. Self-reported snoring, maternal obesity and neck circumference as risk factors for pregnancy-induced hypertension and preeclampsia. Respiration. 2008; 76:33–39.
25. Facco FL, Grobman WA, Kramer J, Ho KH, Zee PC. Self-reported short sleep duration and frequent snoring in pregnancy: impact on glucose metabolism. Am J Obstet Gynecol. 2010;203:e1–e5.
26. Bjorksten B. Disease outcomes as a consequence of environmental influences on the development of the immune system. CurrOpin Allergy Clin Immunol. 2009;9:185–189.
27. Strange LB, Parker KP, Moore ML, Strickland OL, Bliwise DL. Disturbed sleep and preterm birth: a potential relationship? Clin Exp Obstet Gynecol. 2009;36:166–168.
28. Katerina Micheli, IoannisKomninos, EmmanouelBagkeris, Theano Roumeliotaki, AntonisKoutis, ManolisKogevinas and Leda Chatzia. Sleep Patterns in Late Pregnancy and Risk of Preterm Birth and Fetal Growth Restriction. Epidemiology. 2011;22:738-744.
29. Bourjeily G, Raker CA, Chalhoub M, Miller MA. Sleep disordered breathing symptoms in pregnancy and adverse pregnancy and fetal outcomes. Eur Respir J. 2010.
30. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006; 444:860–867.
31. Ford ES. The metabolic syndrome and C-reactive protein, fibrinogen, and leukocyte count: findings from the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2003;168:351–358.
32. Haffner SM. The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol. 2006;97:3A–11A.
33. Kowall J, Clark G, Nino-Murcia G, Powell N. Precipitation of obstructive sleep apnea during pregnancy. Obstet Gynecol. 1989;74:453–455.
34. Sahota PK, Jain SS, Dhand R. Sleep disorders in pregnancy. CurrOpinPulm Med. 2003;9:477–483.
35. Artal R, Wiswell R, Romem Y, Dorey F. Pulmonary responses to exercise in pregnancy. Am J Obstet Gynecol. 1986;154:378–383.
36. Schoenfeld A, Ovadia Y, Neri A, Freedman S. Obstructive sleep apnea (OSA)-implications in maternal-fetal medicine. A hypothesis. Med Hypotheses. 1989;30:51–54.
