Molecular mechanism regulating gene activity in response to varying levels of oxygen
Janet Sugantha M
Associate Professor, Department of Physiology, KAPV Govt. Medical College, Tiruchirapalli, Tamil Nadu, India
Abstract: The fundamental importance of Oxygen is well known for centuries, but how cells adapt to changes in Oxygen levels remains a mystery. William Kaelin, Jr., Sir Peter Ratcliffe, and Gregg Semenza unravelled this mystery for which they were awarded the Nobel prize for Physiology or Medicine in 2019. They discovered how cells can sense and adapt to changing oxygen availability. They identified the molecular machinery that regulates the activity of genes in response to different levels of Oxygen. In this article we will see a detailed account of the molecular mechanism of the cells in response to Normoxia and Hypoxia. This mechanism is one of life’s most essential adaptive processes. This discovery has established the basis of our understanding of how oxygen levels affect metabolism of the cells and physiological function. It has also paved the way for promising new strategies to fight anaemia, cancer and many other diseases.
Keywords: erythropoietin, hypoxia, metabolism, oxygen
Corresponding Author ::
Dr. Janet Sugantha M,
Associate Professor,
Department of Physiology,
KAPV Govt. Medical College, Tiruchirapalli, Tamilnadu. Telephone: +91 99404 83874
E-mail: [email protected]
Introduction: The Nobel Prize in Physiology or Medicine for 2019 is awarded to William Kaelin, Jr., Sir Peter Ratcliffe, and Gregg Semenza. Oxygen is important to sustain life and the molecular mechanism by which cells adapt to variations in oxygen supply was elucidated by the 3 Nobel Laureates.
Background: In the early 1990’s, Gregg Semenza identified and in 1995 he purified and cloned a transcription factor that regulates the response to Oxygen. This transcription factor was namedas Hypoxia Inducible Factor (HIF), and it comprises of two subunits: an oxygen-sensitive portion called HIF-1α, and a non-oxygen-regulated protein called ARNT.
In 1995, William Kaelin, Jr isolated the first full-length clone of the von Hippel-Lindau tumour suppressor gene. He proved that it could suppress the growth of tumour in VHL mutant tumourigenic cell lines.
In 1999, Ratcliffe proved that there was an association between VHLand HIF-1α. He also demonstrated VHL regulated HIF-1α post-translational and oxygen-sensitive degradation.
William G. Kaelin Jr., Sir Peter J. Ratcliffe and Gregg L. Semenza discovered how cells can sense and adapt to changes in the availability of Oxygen. They identified molecular machinery that regulates the activity of genes in response to different concentrations of Oxygen.
Oxygen sensing mechanism: Oxygen constitutes 21 percent of Earth’s atmosphere. Oxygen is essential for animal life; the mitochondria in the animal cells utilizes Oxygen to convert food into energy. Otto Warburg, who was awarded the Nobel Prize in Physiology or Medicine in 1931 established that this conversion is an enzymatic process.
Chemoreceptors: Now let us look into the mechanisms which ensure an adequate supply of oxygen to the tissue level . The carotid bodies present in the large blood vessels in the neck contain chemoreceptors that sense the concentration of oxygen in the blood.
Corneille Heymans was awarded the Nobel Prize in Physiology or Medicine in 1938, for establishing the role of chemoreceptors in the carotid body in the regulation of respiration. The carotid bodies are important for the rapid increase in ventilation due to hypoxia.
Erythropoietin: The other adaptations to hypoxia include an increase in levels of the hormone Erythropoietin (EPO), released by the kidney which in turn stimulates Erythropoiesis. Hypoxia is the most potent stimulus for erythropoiesis.
The Erythropoietin gene was studied by Gregg Semenza and this gene is regulated by varying levels of Oxygen. By using gene-modified mice, specific DNA fragments situated close to the EPO gene were shown to mediate the response to hypoxia.
Sir Peter Ratcliffe also did research on Oxygen-dependent regulation of the Erythropoietin gene. What was interesting is that both the researchers found that the Oxygen sensing mechanism was present in virtually all tissues and it was not limited to the kidney cells where Erythropoietin is produced. Thus the mechanism was general and functional in many cells of the body.
Hypoxia inducible factor: Semenza cultured liver cells and discovered a protein complex which binds to the identified DNA segment in an oxygen-dependent manner.
This complex was named the hypoxia-inducible factor (HIF) . Semenza purified the HIF complex and identified the genes encoding HIF.
HIF consists of two different DNA-binding proteins or transcription factors.
(a) oxygen-sensitive portion called HIF-1α and
(b) non-oxygen-regulated protein called ARNT (Aryl hydrocarbon Receptor Nuclear Translocator)
When the oxygen concentration in the tissues are high, the cellular concentration of HIF-1α will be less. But, when the concentration of oxygen falls, the amount of HIF-1α increases and it will go and bind to and regulate the EPO gene as well as other genes . Many researchers have established that HIF-1α is protected from degradation in hypoxia.
In 2004, the Nobel Prize winners in Chemistry, Aaron Ciechanover, Avram Hershko and Irwin Rosediscovered Proteasome, a cellular machinery which degrades older and unwanted proteins.
At normal oxygen concentration, the proteasome degrades HIF-1α. A small peptide ubiquitin, is added to the HIF-1α protein. Ubiquitin functions as a tag for proteins destined for degradation in the proteasome. How ubiquitin binds to HIF-1α in an oxygen-dependent manner remained a mystery.
Von Hippel Lindau’s disease: William Kaelin, Jr. was doing research in an inherited syndrome, von Hippel-Lindau’s disease (VHL disease). This genetic disease leads to an increased risk of cancers in families with inherited mutations in the VHL gene. Kaelin showed that the VHL gene encodes a protein that prevents the development of cancer. Kaelin also found that cancer cells lacking a functional VHL gene express abnormally high levels of hypoxia-regulated genes.
On the other hand when the VHL gene was reintroduced into the cancer cells, normal levels were restored. This finding gave a clue that VHL was important in mediating responses to hypoxia.
Other research studies have shown that VHL plays a role in tagging unwanted proteins with ubiquitin(3), marking them forproteasomal degradation. Ratcliffe and his coworkers made a key discovery: at normal Oxygen concentration VHL can physically interact with HIF-1α and lead to its degradation.
Interaction of VHL and HIF-1α: Kaelin and Ratcliffe found that the Oxygen sensing element is located in a specific portion of the HIF-1αprotein domain. In 2001, the researchers showed that under normal oxygen concentration, hydroxyl groups are added at two specific positions in HIF-1α (Figure 1). This protein modification is called prolyl hydroxylation. This is an important step which allows VHL to recognize and bind to HIF-1α . This elucidates the importance of oxygen sensitive enzymes (prolylhydroxylases) in rapid HIF-1α degradation(5), under normal cellular Oxygen levels.
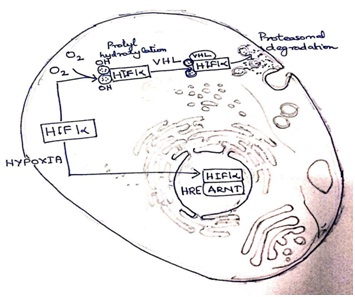
During hypoxia, [Refer figure 1] HIF-1α does not undergo degradation and it is translocated into the nucleus in association with ARNT. HIF/ ARNT dimer binds to specific DNA sequences (HRE) of hypoxia-regulated genes.
Conclusion: Thanks to the Nobel Laureates, we now have a clear understanding about how different oxygen concentrations regulate fundamental physiological processes. Oxygen regulated mechanism helps cells to adapt their metabolism to low oxygen concentration: for example, in our muscles during severe exercise and during acclimatization at high altitudes. Other examples of adaption controlled by oxygen sensing include the formation of new blood vessels and the production of erythrocytes. Oxygen sensing plays an important role in our immune system and during foetal development.
Oxygen sensing molecular machinery helps to understand the pathological basis of a large number of diseases including cancers. For example, patients with chronic renal failure often suffer from severe anemia due to decreased Erythropoietin expression. Increased levels of HIF are seen in many cancers as well as in some cardiovascular diseases including myocardial infarction, pulmonary hypertension and stroke.
This Nobel Prize winning discovery has also paved the path for developing novel strategies to fight anemia, cancer and various other diseases.
Acknowledgements: Nil.
Conflict of Interest: Nil.
References:
1. Semenza, G.L, Nejfelt, M.K., Chi, S.M. & Antonarakis, S.E. (1991). Hypoxia-inducible nuclear factors bind to an enhancer element located 3’ to the human erythropoietin gene. Proc Natl Acad Sci USA, 88, 5680-5684.
2. Wang, G.L., Jiang, B.-H., Rue, E.A. & Semenza, G.L. (1995). Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA, 92, 5510-5514.
3. Jaakkola, P., Mole, D.R., Tian, Y.-M., Wilson, M.I., Gielbert, J., Gaskell, S.J., von Kriegsheim, A., Heberstreit, H.F., Mukherji, M., Schofield, C.J., Maxwell, P.H., Pugh, C.W. & Ratcliffe, P.J. (2001). Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science, 292, 468-472.
4. Maxwell, P.H., Wiesener, M.S., Chang, G.-W., Clifford, S.C., Vaux, E.C., Cockman, M.E., Wykoff, C.C., Pugh, C.W., Maher, E.R. & Ratcliffe, P.J. (1999). The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature, 399, 271-275.
5. Ivan, M., Kondo, K., Yang, H., Kim, W., Valiando, J., Ohh, M., Salic, A., Asara, J.M., Lane, W.S. & Kaelin Jr., W.G. (2001) HIFa targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science, 292, 464-468.
6. https://www.nobelprize.org (accessed on November 22, 2019).
